Case Study: FNA of pancreatic cystic lesion
Introduction
High equipment costs have confined EUS mainly to hospital settings, limiting access. The EndoSound Vision System™ (EVS™) technology transforms any endoscope into a fully functioning endoscopic ultrasound (EUS) system. We use the EVS for Echo-EGD, defined as diagnostic EGD enhanced with ultrasound for many imaging-only procedures in our ASC. This paper describes the EVS’s additional capabilities of fine needle aspiration (FNA) which is traditionally performed in a hospital setting.
The EVS is cost-effective and opens new possibilities for EUS across care settings. EndoSound provides needles that fit the length of gastroscopes enabling the capability of FNA in the ASC. Thus, given the cost of the traditional EUS equipment this is an exciting innovation to perform EUS procedures in smaller surgery centers.
Below, is a case using the EVS to evaluate the symptom and perform FNA of a pancreatic cyst.
Patient History
81-year-old male with history of lymphoplasmacytic lymphoma and pancreatic surgery for pancreatic cyst many years ago presented with upper abdominal pain. Details of prior surgery is not known.
The Procedure
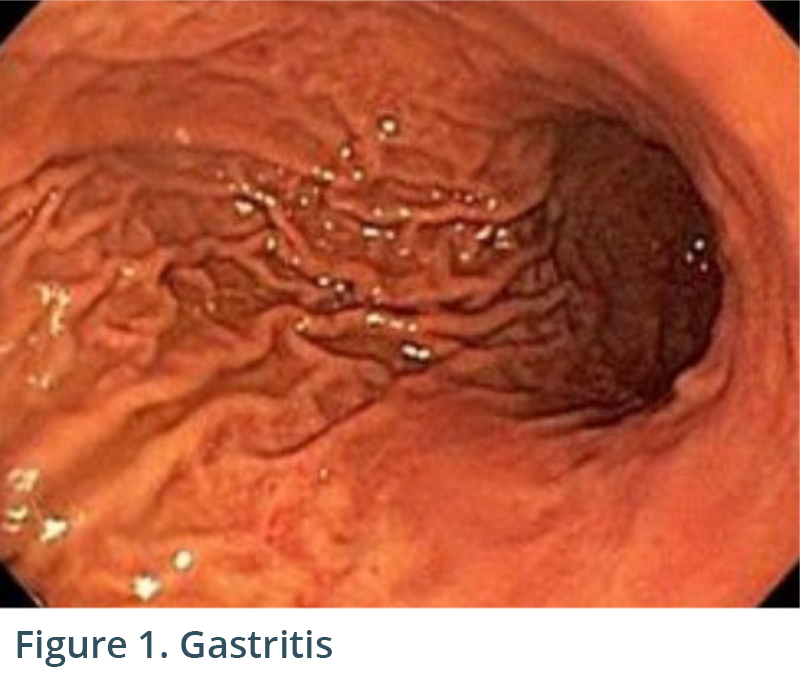
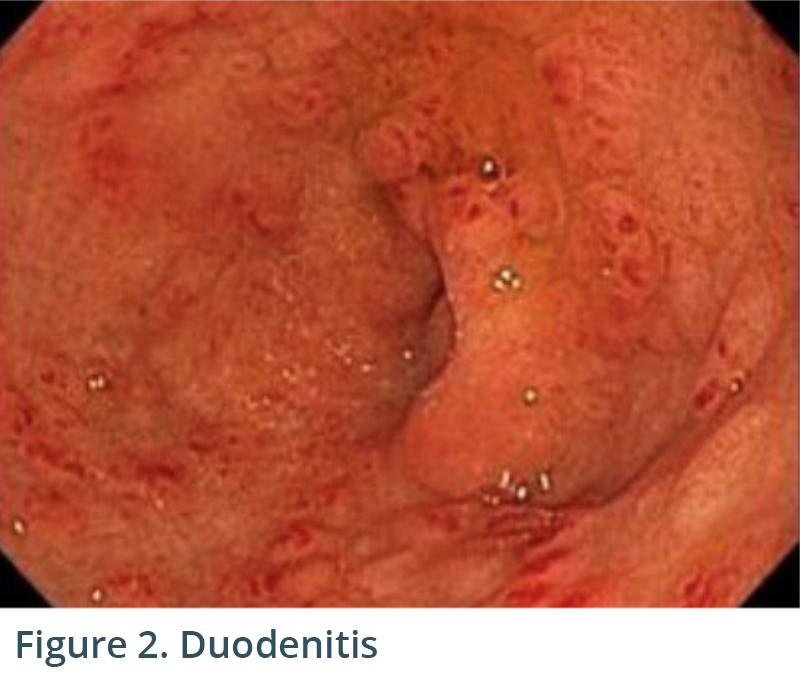
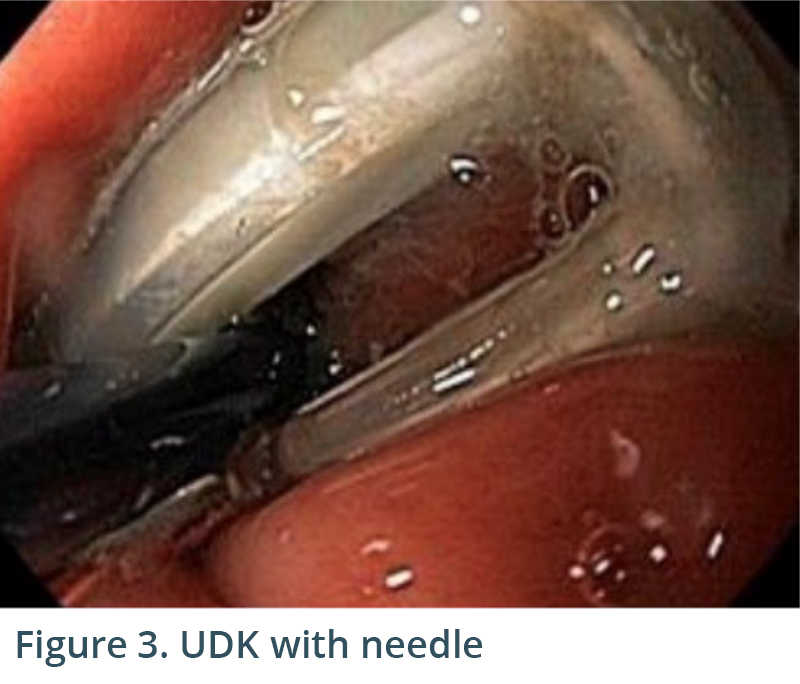
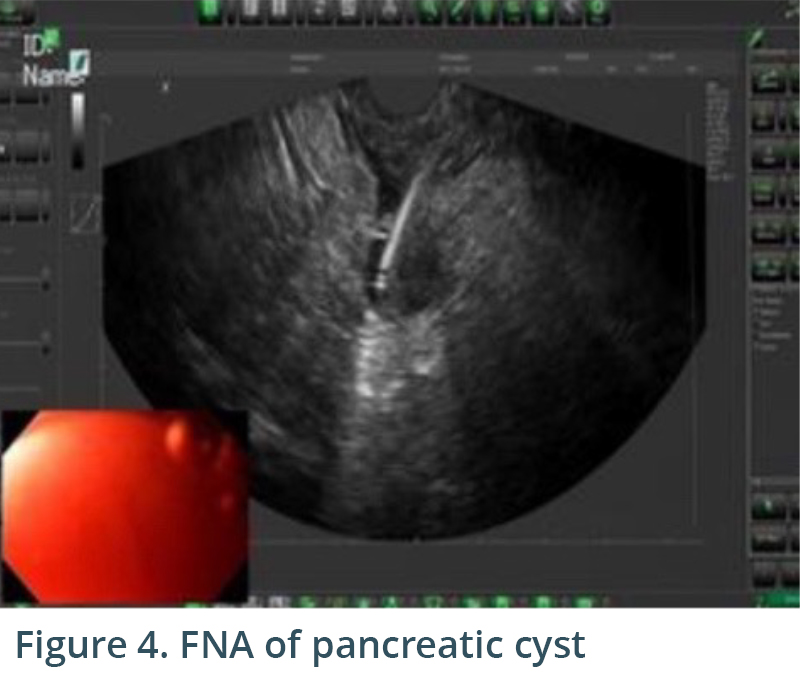
Endoscopic exam revealed gastritis (Fig 1) and duodenitis in duodenal bulb (Fig 2). Pancreatic parenchyma was without mass, and there was no dilation of main pancreatic duct. There was a cyst in head of pancreas measuring 4.7 x 2.5 mm and another in the body of pancreas measuring 5.1 x 4.3 mm. Both cysts were without mural nodule or associated mass and were too small for meaningful FNA. Tail of pancreas had a 14.6 x 12.9 mm cyst without mural nodule or mass status post FNA with 22G needle (Figs 3, 4) with aspiration of approximately 1.5-2 cc of viscous/straw colored fluid sent for analysis. Other etiologies of abdominal pain such as gallstones and biliary causes were ruled out during EUS (Figs 5,6).
Cytology was negative for malignancy. CEA was 3008 and amylase 105. Molecular test was negative for GNAS, KRAS and LOH and concluded that there was a 97% probability that the cyst would remain benign over the next three years. Duodenal biopsy revealed gastric heterotopia, and gastric biopsy was negative for helicobacter pylori. It was determined that the most likely etiology of his abdominal pain was gastric heterotopia versus Zanubrutinib which he was taking for his lymphoma.


Conclusion
The EVS allowed both visualization/fine needle aspiration of pancreatic cyst lesion and the work-up of patient’s abdominal pain in an ASC setting. With the supply of the FNA needles for the EVS specifically, it is a complete system allowing FNA or fine needle biopsy (FNB) in the ASC. Moreover, Echo-EGD can be utilized as a first-line investigation, bypassing other imaging modalities and streamlining the diagnostic process (Sahai, et al., 2000; Chang et al., 2010). By making EUS capabilities more accessible and affordable, we are revolutionizing the standard of care in endoscopy.
References:
Sahai et al., Gastrointest Endosc 2000;52:153-9. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia.
Chang et al., Gastrointest Endosc 2010; 72: 967-74. EUS compared with endoscopy plus transabdominal US in the initial diagnostic evaluation of patients with upper abdominal pain.